Rameem Medical Company
Exhibitors
Information
Rameem Medical Company is a Saudi Arabia–based manufacturer specializing in orthopedic implants. The company is headquartered in Al Khobar, Saudi Arabia. Rameem positions itself as the first local manufacturer of titanium orthopedic implants in the Kingdom, contributing directly to Saudi Arabia’s growing medical device industry and supporting the national drive for industrial localization under Saudi Vision 2030.
Rameem’s vision is to become the leading orthopedic implant manufacturer in Saudi Arabia by 2030. Its mission centers on providing high-quality, safe, and technologically advanced medical devices through innovation, research collaboration, and adherence to international standards. The company works closely with hospitals, universities, and government agencies to develop products that meet both local and global medical needs, while nurturing Saudi talent in high-precision manufacturing and biomedical engineering.
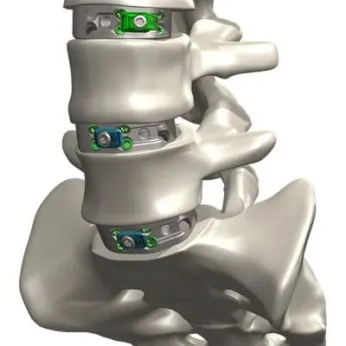
The company manufactures a comprehensive range of orthopedic implants, including systems for the upper and lower limbs, plates, screws, and fixation devices. These products are produced using biocompatible materials such as medical-grade titanium, ensuring patient safety and long-term durability. Rameem’s product portfolio is continuously expanding to include new business lines such as spine, arthroplasty, maxillofacial, and dental implants, reflecting its ambition to cover the full spectrum of orthopedic and reconstructive care.
Rameem has strengthened its market position through strategic partnerships, most notably with Zahrawi Group, one of the region’s leading medical distributors. Under this agreement, Zahrawi serves as the exclusive distributor for Rameem’s trauma portfolio in Saudi Arabia. This collaboration enhances Rameem’s access to hospitals and healthcare institutions across the Kingdom, enabling broader market penetration and stronger brand recognition.
Despite its achievements, Rameem faces challenges typical of advanced manufacturing in the medical field. These include sourcing high-quality raw materials such as medical-grade titanium, achieving and maintaining certifications like ISO 13485, and complying with Saudi Food and Drug Authority (SFDA) requirements. However, the company views these challenges as opportunities for growth and continuous improvement, aiming to position itself as a regional hub for medical implant innovation and export.
Looking ahead, Rameem Medical Company’s strategy focuses on scaling production capacity, diversifying product lines, and entering new global markets. By aligning with Saudi Vision 2030’s industrial and healthcare objectives, Rameem not only supports the Kingdom’s self-sufficiency in medical manufacturing but also contributes to raising the overall standard of healthcare in the region. With a commitment to “quality first” and a patient-centric approach, Rameem stands as a pioneering force in the advancement of Saudi Arabia’s medical device industry.